Mucograft®
In stock
Only %1 left
SKU
50010
Description
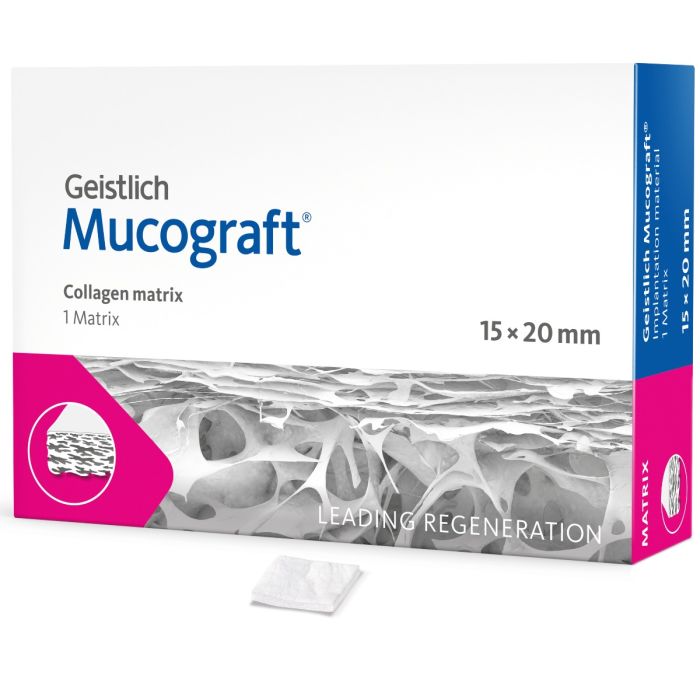
Geistlich Mucograft® is a unique 3D collagen matrix designed specifically for soft-tissue regeneration in the oral cavity1. It is indicated for gaining keratinised tissue2,3,4 and for recession coverage5,6.
Geistlich Mucograft® provides an alternative to autogenous soft-tissue grafts2,4,5,6. Painful harvesting of tissue is avoided, benefiting patients and clinicians alike.
Leading clinicians rely on Geistlich Mucograft®:
- No harvest-site morbidity2,3,5-7.
- Less pain compared with autogenous grafts2,4.
- Reduced surgical chair-time compared with autogenous grafts2-6.
- Natural soft-tissue colour and texture match5,8,9.
- Early vascularisation and good soft-tissue ingrowth10,11.
- Excellent wound healing, also in open situations2.
- Easy handling2 and application in a dry state.
- Geistlich Mucograft® consists of two structures: the compact structure provides stability while allowing open healing; the spongy structure supports blood clot stabilisation and ingrowth of soft-tissue cells.
References:
1. Biocompatibility according to ISO 10993-1:2001. Data on file, Geistlich Pharma AG, Wolhusen, Switzerland.
2. Sanz M, et al.: J Clin Periodontol 2009; 36(10): 868-76.
3. Konter U, et al.: Deutsche Zahnärztliche Zeitschrift 2010; 65: 723-30.
4. Lorenzo R, et al.: Clin Oral Impl Res 2012; 23(3): 316-24.
5. McGuire MK & Scheyer ET: J Periodontol 2010; 81(8): 1108-17.
6. Cardaropoli D, et al.: J Periodontol 2012; 83(3): 321-28.
7. Herford AS, et al.: J Oral Maxillofac Surg 2010; 68(7): 1463-70.
8. Thoma, D, et al.: J Clin Periodontol 2012; 39(2): 157-65.
9. Nevins M, et al.: Int J Periodontics Restorative Dent 2011; 31(4): 367-73.
10. Ghanaati S, et al.: Biomed Mater. 2011 Feb; 6(1): 015010.
11. Rocchietta I, et al.: Int J Periodontics Restorative Dent 32(1): e34-40.